Periodic Table With Full Detail
Published Jan 16, 2022 | By Admin

Here we have provided you the list of Periodic table elements that you can see and can learn about Periodic table. Moreover we have provided you the detail of periodic table under periodic table chart that you can see and gain interesting information about periodic table. 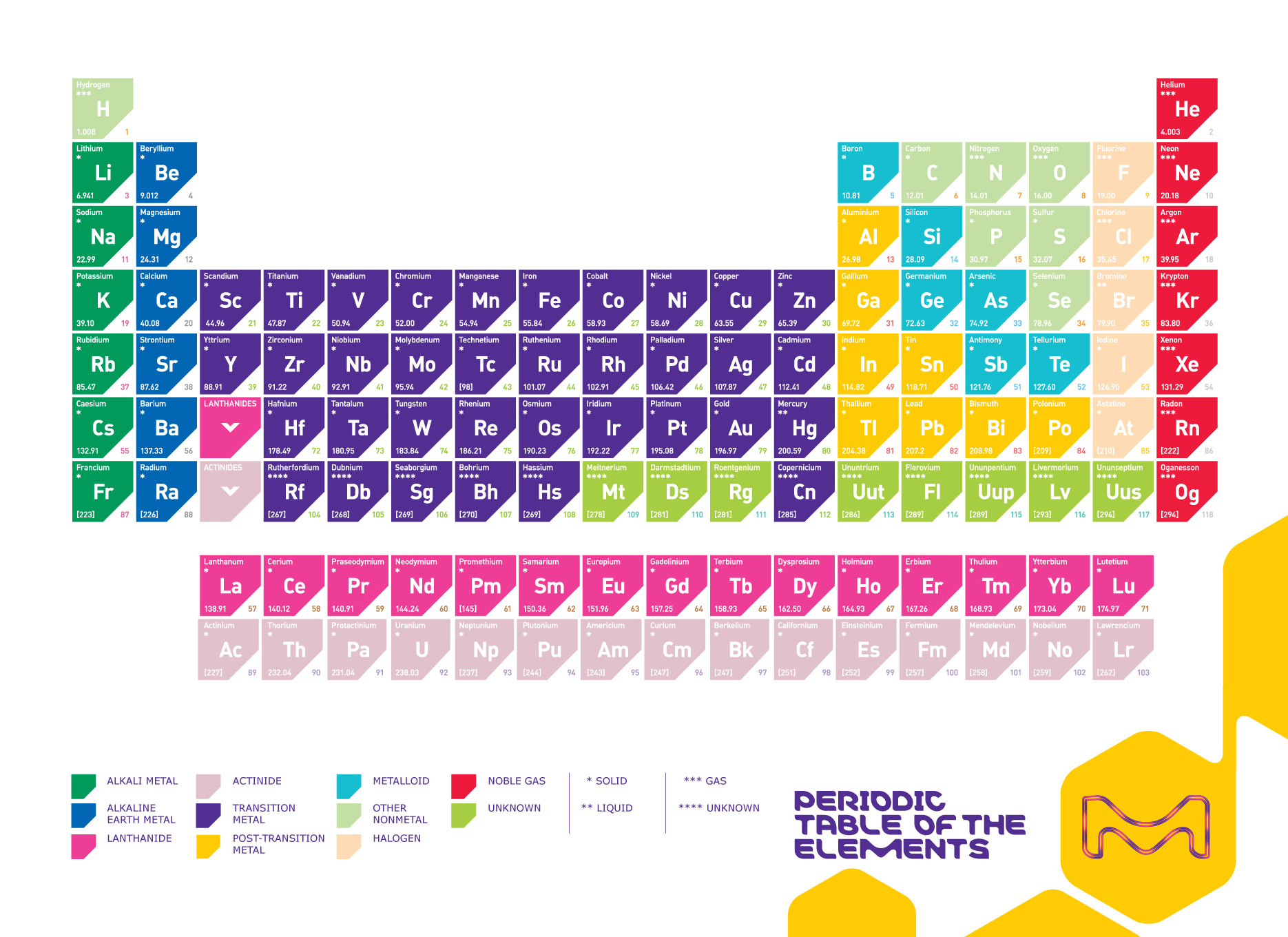
Periodic Table Chart
| Element Name | Symbol | Atomic Number | Electronegativity (χ) |
|---|---|---|---|
| Actinium | Ac | 89 | 1.1 |
| Aluminum | Al | 13 | 1.61 |
| Americium | Am | 95 | 1.3 |
| Antimony | Sb | 51 | 2.05 |
| Argon | Ar | 18 | |
| Arsenic | As | 33 | 2.18 |
| Astatine | At | 85 | 2.2 |
| Barium | Ba | 56 | 0.89 |
| Berkelium | Bk | 97 | 1.3 |
| Beryllium | Be | 4 | 1.57 |
| Bismuth | Bi | 83 | 2.02 |
| Bohrium | Bh | 107 | |
| Boron | B | 5 | 2.04 |
| Bromine | Br | 35 | 2.96 |
| Cadmium | Cd | 48 | 1.69 |
| Calcium | Ca | 20 | 1 |
| Californium | Cf | 98 | 1.3 |
| Carbon | C | 6 | 2.55 |
| Cerium | Ce | 58 | 1.12 |
| Cesium | Cs | 55 | 0.79 |
| Chlorine | Cl | 17 | 3.16 |
| Chromium | Cr | 24 | 1.66 |
| Cobalt | Co | 27 | 1.88 |
| Copper | Cu | 29 | 1.9 |
| Curium | Cm | 96 | 1.3 |
| Darmstadtium | Ds | 110 | |
| Dubnium | Db | 105 | |
| Dysprosium | Dy | 66 | 1.22 |
| Einsteinium | Es | 99 | 1.3 |
| Erbium | Er | 68 | 1.24 |
| Europium | Eu | 63 | |
| Fermium | Fm | 100 | 1.3 |
| Fluorine | F | 9 | 3.98 |
| Francium | Fr | 87 | 0.7 |
| Gadolinium | Gd | 64 | 1.2 |
| Gallium | Ga | 31 | 1.81 |
| Germanium | Ge | 32 | 2.01 |
| Gold | Au | 79 | 2.54 |
| Hafnium | Hf | 72 | 1.3 |
| Hassium | Hs | 108 | |
| Helium | He | 2 | |
| Holmium | Ho | 67 | 1.23 |
| Hydrogen | H | 1 | 2.2 |
| Indium | In | 49 | 1.78 |
| Iodine | I | 53 | 2.66 |
| Iridium | Ir | 77 | 2.2 |
| Iron | Fe | 26 | 1.83 |
| Krypton | Kr | 36 | 3 |
| Lanthanum | La | 57 | 1.1 |
| Lawrencium | Lr | 103 | |
| Lead | Pb | 82 | 2.33 |
| Lithium | Li | 3 | 0.98 |
| Lutetium | Lu | 71 | 1.27 |
| Magnesium | Mg | 12 | 1.31 |
| Manganese | Mn | 25 | 1.55 |
| Meitnerium | Mt | 109 | |
| Mendelevium | Md | 101 | 1.3 |
| Mercury | Hg | 80 | 2 |
| Molybdenum | Mo | 42 | 2.16 |
| Neodymium | Nd | 60 | 1.14 |
| Neon | Ne | 10 | |
| Neptunium | Np | 93 | 1.36 |
| Nickel | Ni | 28 | 1.91 |
| Niobium | Nb | 41 | 1.6 |
| Nitrogen | N | 7 | 3.04 |
| Nobelium | No | 102 | 1.3 |
| Oganesson | Uuo | 118 | |
| Osmium | Os | 76 | 2.2 |
| Oxygen | O | 8 | 3.44 |
| Palladium | Pd | 46 | 2.2 |
| Phosphorus | P | 15 | 2.19 |
| Platinum | Pt | 78 | 2.28 |
| Plutonium | Pu | 94 | 1.28 |
| Polonium | Po | 84 | 2 |
| Potassium | K | 19 | 0.82 |
| Praseodymium | Pr | 59 | 1.13 |
| Promethium | Pm | 61 | |
| Protactinium | Pa | 91 | 1.5 |
| Radium | Ra | 88 | 0.9 |
| Radon | Rn | 86 | |
| Rhenium | Re | 75 | 1.9 |
| Rhodium | Rh | 45 | 2.28 |
| Roentgenium | Rg | 111 | |
| Rubidium | Rb | 37 | 0.82 |
| Ruthenium | Ru | 44 | 2.2 |
| Rutherfordium | Rf | 104 | |
| Samarium | Sm | 62 | 1.17 |
| Scandium | Sc | 21 | 1.36 |
| Seaborgium | Sg | 106 | |
| Selenium | Se | 34 | 2.55 |
| Silicon | Si | 14 | 1.9 |
| Silver | Ag | 47 | 1.93 |
| Sodium | Na | 11 | 0.93 |
| Strontium | Sr | 38 | 0.95 |
| Sulfur | S | 16 | 2.58 |
| Tantalum | Ta | 73 | 1.5 |
| Technetium | Tc | 43 | 1.9 |
| Tellurium | Te | 52 | 2.1 |
| Terbium | Tb | 65 | |
| Thallium | Tl | 81 | 1.62 |
| Thorium | Th | 90 | 1.3 |
| Thulium | Tm | 69 | 1.25 |
| Tin | Sn | 50 | 1.96 |
| Titanium | Ti | 22 | 1.54 |
| Tungsten | W | 74 | 2.36 |
| Ununbium | Uub | 112 | |
| Ununhexium | Uuh | 116 | |
| Ununpentium | Uup | 115 | |
| Ununquadium | Uuq | 114 | |
| Ununseptium | Uus | 117 | |
| Ununtrium | Uut | 113 | |
| Uranium | U | 92 | 1.38 |
| Vanadium | V | 23 | 1.63 |
| Xenon | Xe | 54 | 2.6 |
| Ytterbium | Yb | 70 | |
| Yttrium | Y | 39 | 1.22 |
| Zinc | Zn | 30 | 1.65 |
| Zirconium | Zr | 40 | 1.33 |
History of Periodic table
If we look at the history of the Periodic Table, only nine elements were discovered before 1800. But then in eighteen hundred and thirty-four elements will be discovered. Then in eighteen hundred and seventy, sixty-eight elements will be discovered. And then one hundred and eighteen elements will be discovered in nineteen hundred and five. Ninety-two of the one hundred and eighteen elements were natural. And the remaining twenty elements were made in the laboratory. But scientists are still trying to discover new elements that may be an element that is out of sight.
As time went on, when one hundred and eighteen elements were discovered, he had to study them and the property of each element matched that of the other elements. And it was difficult to study again, then people would be forced and they said that now we will make a table in which all the elements will be arranged so that it is easy for them to study. And so the periodic table came into being.






